Dimethyl sulfoxide
Popularly knowns by its acronym DMSO, is an organosulfur compound. It is an aprotic polar solvent, a mild oxidant, and a common ligand in co-ordination chemistry. Its versatility lies in the fact that it can dissolve polar as well as non-polar solutes and hence it is a special solvent in organic syntheses. Its utility is further due to its low cost, low toxicity, and stability.
Manufacture
It is manufactured by oxidation of dimethyl sulfide by either oxygen or nitrogen dioxide.
| Synonyms |
Dimethyl sulfur oxide
Methylsulfinylmethane |
| CAS no. |
67-68-5 |
| EINECS no. |
200-664-3 |
| Molecular formula |
C2H6OS |
| Molecular weight |
78.13 |
| Structure |
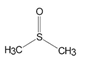 |
SPECIFICATIONS
| Test |
Unit |
Specifications |
| Crystallization point |
°C |
Min 18.10 |
| Acid value |
mg KOH/g |
Max 0.03 |
| Transmittance at 400nm |
% |
Min 96.0 |
| Refractive Index at 20 °C |
|
1.4775 – 1.4790 |
| Moisture |
% |
Max 0.1 |
| Purity |
% |
Min 99.90 |
STORAGE
The product is stored at ambient temperature
PACKING
225 kg MS drum